Preserving the specimen is tricky and complicated process and should only be undertaken if you are confident in the process. Anyone keen on learning the essential basics can contact one of the listed museums and a short demonstration of the fixation and specimen setting process will be conducted, as well as supplies of some basic field preservatives, DNA tissue solution, proper tags, etc.
Fixation involves the preservation and stiffening of tissues so that they do not decompose and so that the specimen remains in an easy-to-study posture. Preservation involves the storage of the specimen in a medium that allows for its safe study for many years.
Basic equipment needed is the following:
- A field note book or a reliable field data app on a phone or tablet
- Good quality specimens
- Surgical gloves to protect your hands from the chemicals
- Newspaper to work on
- Plastic fixing tray – a large, shallow plastic container is ideal with a polystyrene layer
- Syringes with needles (large volume syringes with large gauge needles for bigger specimens, as well as smaller syringes and finer needles for slighter body parts and smaller specimens)
- Forceps, scalpels and surgical scissors for collecting tissue
- 70% ethanol for reptiles and amphibians
- 10% formalin ( 1 part 40% Formaldehyde + 9 parts water)
- Archival marker pens
- Tag paper, fine crochet thread and a needle for attaching tags to the specimens
Warnings: Formalin is known to cause cancer and it is important to minimize exposure to these chemicals when using them. Always work in an open space and wear gloves. The use of syringes with needles is another hazard associated with this protocol. Syringes are used to inject specimens with formalin (a carcinogen). Handle syringes carefully. Feel free to place the specimen being fixed in a tray to inject it instead of holding it in your other hand to minimize the risk of sticking yourself with the needle. If stuck by a needle, rinse with cold water for 15 minutes and wash area with soap and water. The final hazard associated with fixation and preservation process is the specimens themselves. Since these are dead animals, they may carry zoonoses and parasites. To minimize the hazard of being exposed to these pathogens, always handle specimens with gloves.
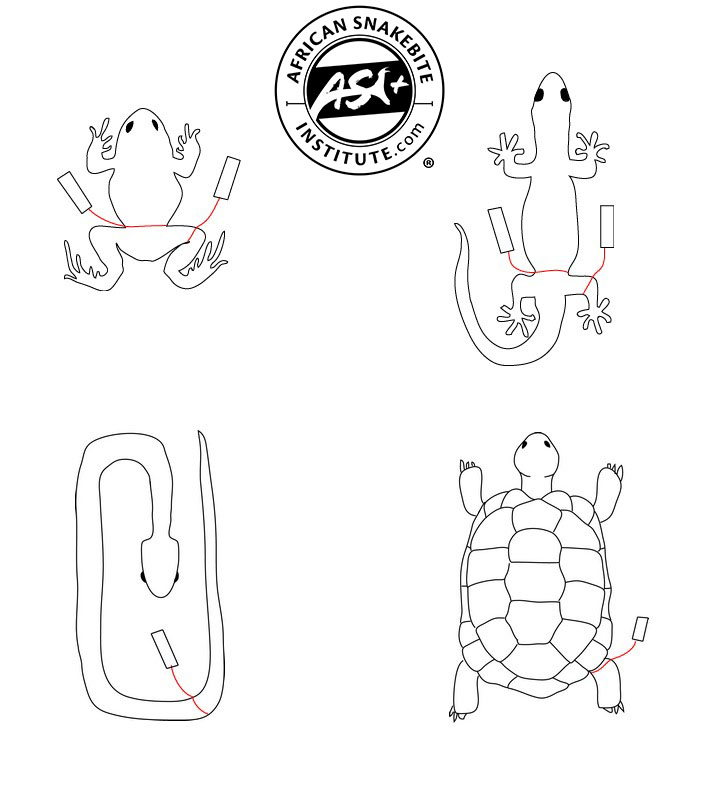
Prior to preserving the specimen, first collect the tissue sample. It should be emphasised that all instruments (scalpel, scissors, forceps) used for collecting tissue samples must be both thoroughly cleaned and sterilised with 70% alcohol (ethanol) before and after taking each sample, and that neither the working surfaces of the instruments nor the tissue being collected must be touched by the worker’s hands. To avoid contamination, putting labels inside the specimen container, while theoretically desirable as a safeguard against loss of data, carries a high risk of contamination and is therefore not recommended. The volume of fixative should be large, at least 20 times the volume of the specimen. The samples must be protected from light at all times and well labelled.